Pulse oximeters need to be improved for people of color, FDA panel says
A U.S. Food and Drug Administration panel suggested on Tuesday that pulse oximeters should be improved because they may not give accurate results to people of color.
Pulse oximeters measure oxygen levels, but studies have shown that the small devices overestimate oxygen levels in people with darker skin, possibly making health disparities in many racial and ethnic groups worse, the FDA said.
"The devices may be less accurate in people with dark skin pigmentation," The U.S. Food and Drug Administration said in a statement in February 2021.
While the FDA advisory panel does not have direct regulatory authority, the FDA itself, which regulates medical devices, takes their opinions very seriously. Some of the panel members who had previously expressed skepticism said they were now convinced that the devices are less accurate in patients with darker skin.
Pulse oximeters use red and infrared light to estimate the oxygen saturation of the blood and pulse rate, and darker skin pigment may absorb some of that light and alter results.

In February 2021, the Centers for Disease Control and Prevention included in its coronavirus clinical guidance that under-detection may happen for people with dark skin.
The devices are one of several tools used by doctors to manage patients. At-home versions of these devices also became popular during the pandemic.
"Clinicians recommending the use of pulse oximeters to patients to monitor health conditions at home, and consumers using OTC pulse oximeters for medical purposes should be aware of the limitations," according to an FDA executive summary from Tuesday's meeting.
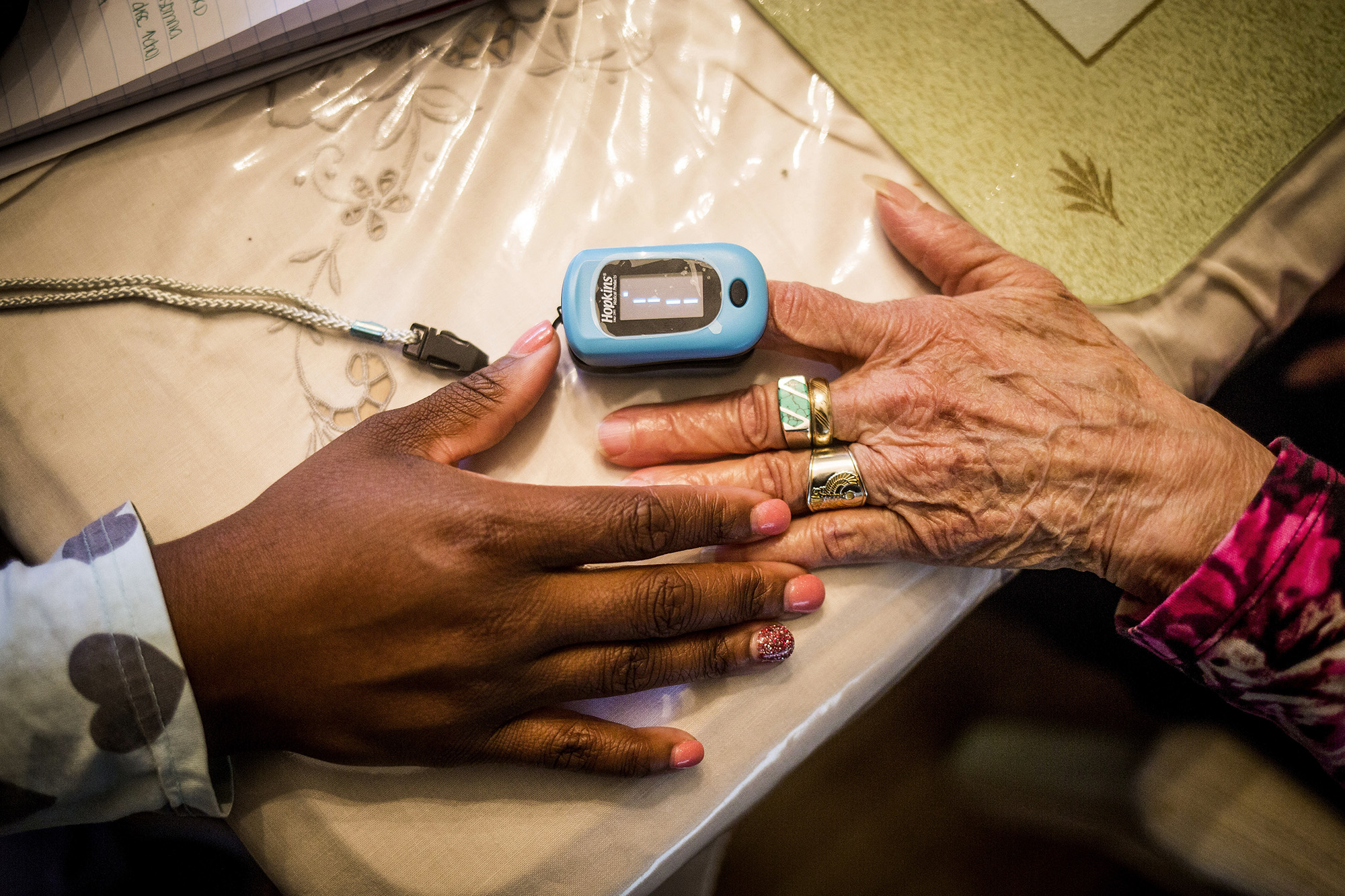
ABC News medical contributor Dr. Darien Sutton said that doctors should advocate for their patients and educate them so they can learn to advocate for themselves.
"We should invest in research to create better devices that are working without bias," Sutton told "Good Morning America" in July.
ABC News' Marlene Lenthang contributed to this report.





