Promising new 15-minute test for coronavirus comes with caveats
As COVID-19 continues to spread, health experts say that efficient testing will be one of the keys to containing the coronavirus. Companies are now scrambling to come up with new, rapid tests -- among them a company called Coris BioConcept, which recently announced a test that can deliver results in just 15 minutes.
Testing availability has been a significant challenge in the U.S., but the situation is slowly getting better. The CDC continues to distribute its test kits to public health laboratories across the nation, while large diagnostics companies like Quest Diagnostics are ramping up to be able to run tens of thousands of tests per day.
The FDA recently updated its policy on diagnostic testing for COVID-19, which helped free up companies to begin distributing their own tests to laboratories, hospitals and other clinical settings. The Coris BioConcept test is not available for at-home use by everyday people, although experts say these types of rapid tests may soon be more widely available.
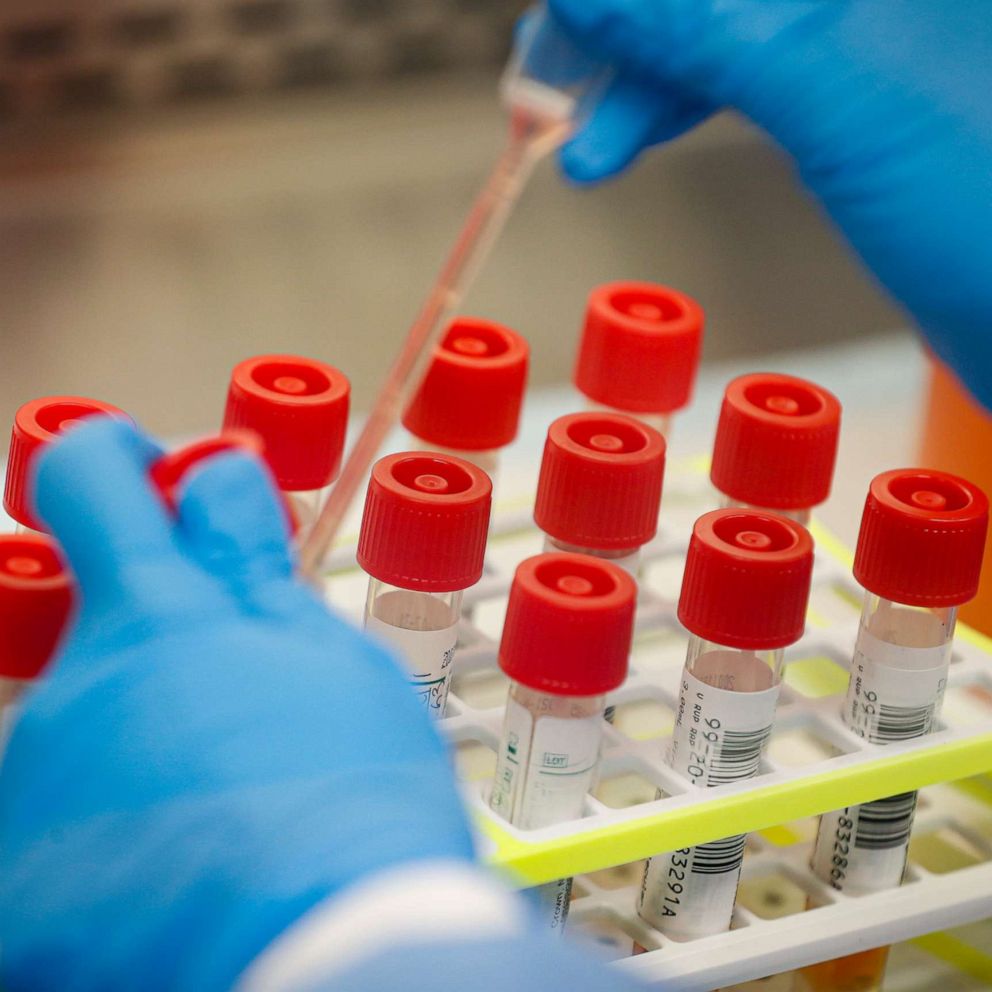
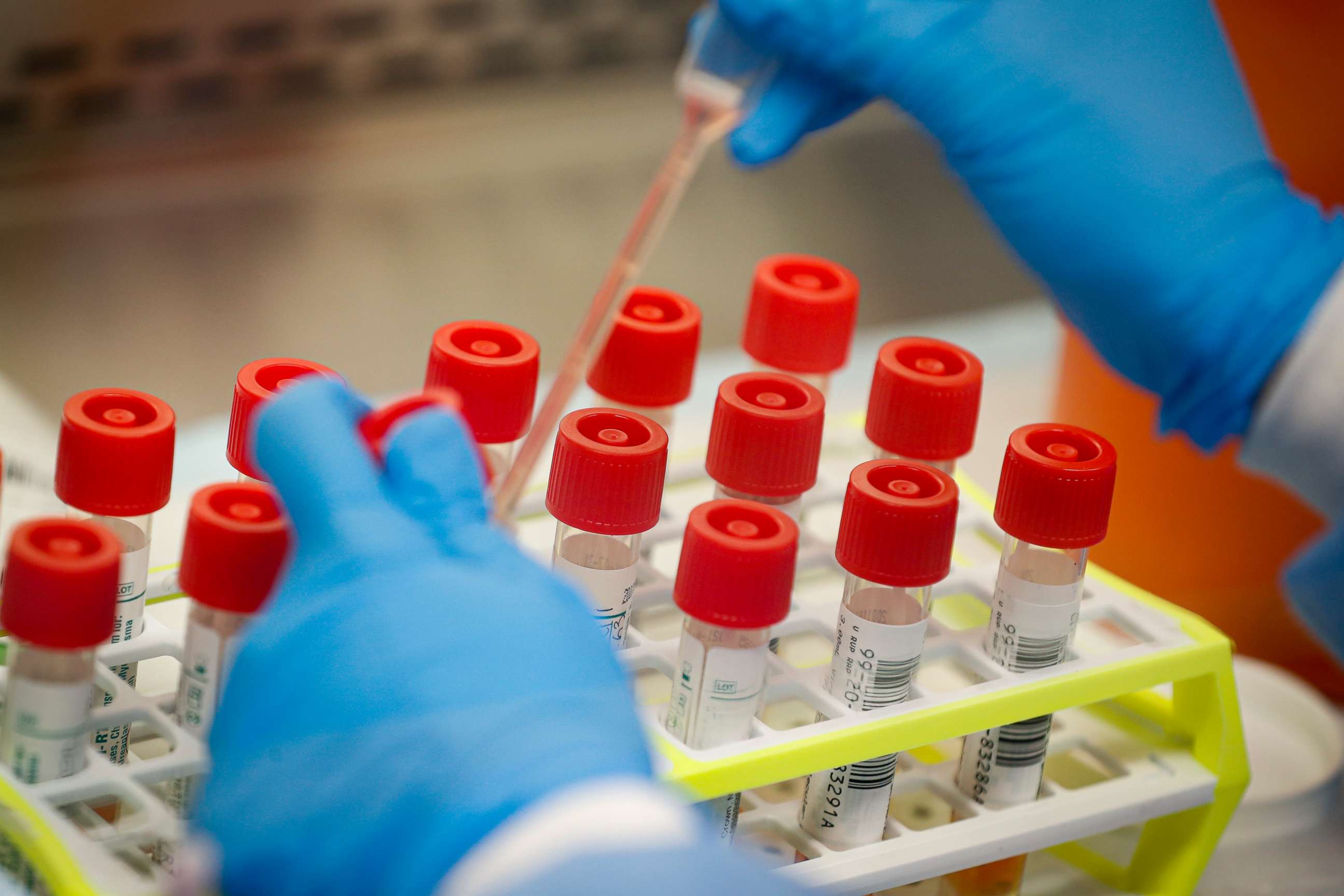
Right now, the majority of COVID-19 test are real-time polymerase chain reaction tests (RT-PCR). These tests “amplify and detect the virus’ genetic material,” according to College of American Pathologist Microbiology Committee Chair Dr. Bobbi Pritt.
It can take anywhere from four to six hours to run an RT-PCR test. In contrast, the test developed by Coris BioConcept takes only 15 minutes. That's because Coris BioConcept's test uses a different technology that detects antigens.
The COVID-19 Ag Respi-Strip test detects viral antigens obtained from a nasal swab sample, as opposed to viral RNA. “This test uses a very common approach called “immunochromatography” or “lateral flow immunoassay,” explained Dr. James Faix, the director of clinical chemistry and immunology at New York City's Montefiore Medical Center.
It works kind of like a pregnancy test.
“The dipstick is a membrane which contains antibody to a part of the CoVID-19 virus, and this antibody is attached to a colored particle," said Faix. "As the diluted sample moves through the membrane, if there is any viral protein in it, the antibody will attach to it, forming a complex. Further down, there is another antibody to the viral protein immobilized on the membrane so the initial antigen-antibody complex will be stuck to this part of the membrane as the sample passes through. This creates the color at the test line, meaning that the result is positive.”
This entire process takes 15 minutes.
But is this test the holy grail?
Experts say not so fast. BioConcept's test is fast, but it's not as good at detecting positive cases of COVID-19 as the PCR tests used today. Specifically, the test's "sensitivity" -- a measure of its ability to detect true positives -- is only 60%.
“It is not very sensitive for detecting the virus in patient specimens," Pritt said. “A sensitivity of 60% means that only 6 out of every 10 cases of COVID-19 will be detected by this test, and 4 will be missed.”
Faix noted that these type of tests “usually have pretty good specificity,” meaning they are unlikely to show a false positive. “But they suffer from generally poor sensitivity -- not identifying most of the people with the disease. This means that a positive result might be helpful for detecting a positive patient but a negative result won’t mean that the patient is not infected.”
Regarding the tests Coris BioConcept ran to determine the sensitivity, Faix said that the two studies cited by the manufacturer were small ones.
“It’s possible that larger studies may show better performance,” he said. “Also, other companies are developing similar assays with different ways of detecting the 'color.' These may enhance the sensitivity of the approach.”

“While I think that this rapid antigen test may have some role in detecting the coronavirus causing COVID-19, the low sensitivity severely limits the utility of this method," said Pritt. "Physicians could use a positive result as evidence that a patient has COVID-19, but they couldn’t use a negative result to rule out the possibility of infection. Instead, all negative results would still have to be confirmed using a more sensitive method such as RT-PCR.”
Nevertheless, this new rapid test is could prove very helpful in expanding U.S. testing capabilities.
“There is no perfect test, and it’s important for physicians to understand the limitations of the tests that they are relying on to make diagnoses,” Pritt said.
The CDC provides guidance regarding who should be tested, but leaves the decision to the discretion of health departments and health care providers. Given that there is no treatment specifically approved for the virus at this time, the CDC recommends that those with mild illness self-isolate and recover at home. Anyone with shortness of breath or fever greater than 100.4F should alert their doctor.
Angela N. Baldwin M.D., M.P.H., is a pathology resident at Montefiore Health Systems in New York City and a contributor to the ABC News Medical Unit.
What to know about the coronavirus:
- How it started and how to protect yourself: Coronavirus explained
- What to do if you have symptoms: Coronavirus symptoms
- Tracking the spread in the U.S. and worldwide: Coronavirus map
Tune into ABC at 1 p.m. ET and ABC News Live at 4 p.m. ET every weekday for special coverage of the novel coronavirus with the full ABC News team, including the latest news, context and analysis.