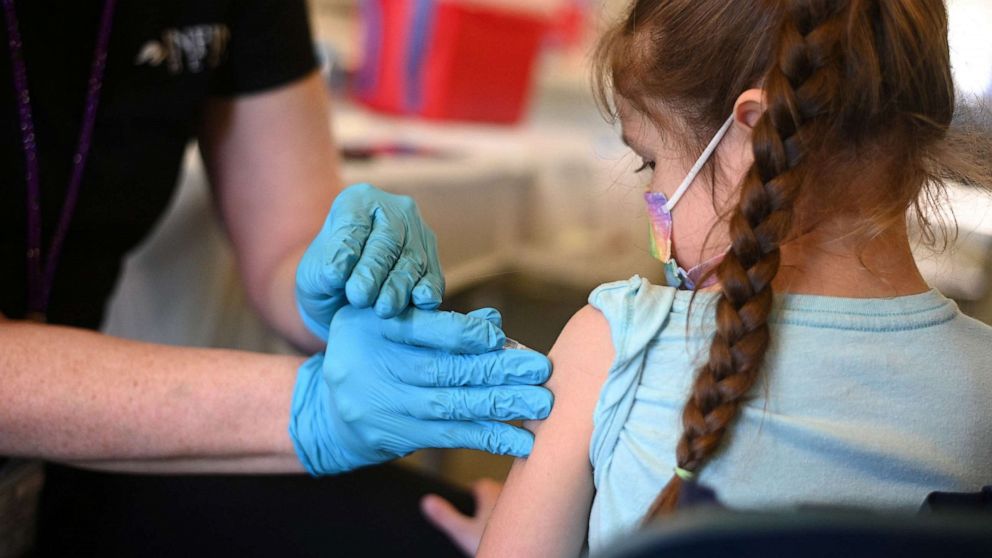
FDA will review Moderna's data on vaccine for young kids without waiting for Pfizer
The Food and Drug Administration will hold meetings to review vaccines for children under 5 in June, it announced Friday, setting the stage for potential authorization soon afterward if the vaccines are deemed safe and effective.
The FDA will convene its panel of independent advisers on June 8, June 21 and June 22 to review and discuss data submitted by Moderna and Pfizer on vaccines for the youngest children, the only age group yet to be eligible for a vaccine. The dates are tentative, the FDA warned, and it's not clear yet which vaccine will be discussed on which days.
But FDA leaders said the agency will be reviewing Moderna’s data for its kid vaccine without unnecessarily delaying authorization until Pfizer submits its data on kids under 5, a move that will be welcome news to some parents who were upset that waiting to authorize the vaccines together would push back the timeline.
The FDA has been considering reviewing the Moderna data for kids under 6, which was submitted for an emergency use authorization on Thursday, alongside Pfizer’s data, which is expected to be submitted in the next few weeks, so that the vaccines could be compared side-by-side.
But on Friday, the FDA's vaccine chief, Dr. Peter Marks, said the agency would only move to do that if both vaccines had fully submitted their applications and were ready for authorization within a week of each other. If there is more of a gap between the two, Marks told the Washington Post in an interview, the FDA would not hold Moderna to review alongside Pfizer.
“We are not going to delay things unnecessarily here,” Marks told the Washington Post. He said he expected at least one of the two vaccines for kids under 5 to be authorized in June, so long as they are found to be safe and effective by the FDA.
On Thursday, FDA Commissioner Robert Califf also told reporters the FDA would not "hold up" the Moderna application to wait for Pfizer.
"Most of the experts that I’ve talked with would say it would be ideal if they could be considered together. But if they don’t come in at the same time, then there’s not going to be a hold up on the Moderna application, just to make it come in at the same time," Califf told reporters Thursday after a hearing on Capitol Hill, in comments that were later confirmed by an FDA spokesperson to ABC News.
And a senior administration official confirmed to ABC News the FDA would act "as expeditiously as possible" to authorize the Moderna vaccine, so long as it meets its standards.
The official said there would be no delay and the application would be judged "on its merits."
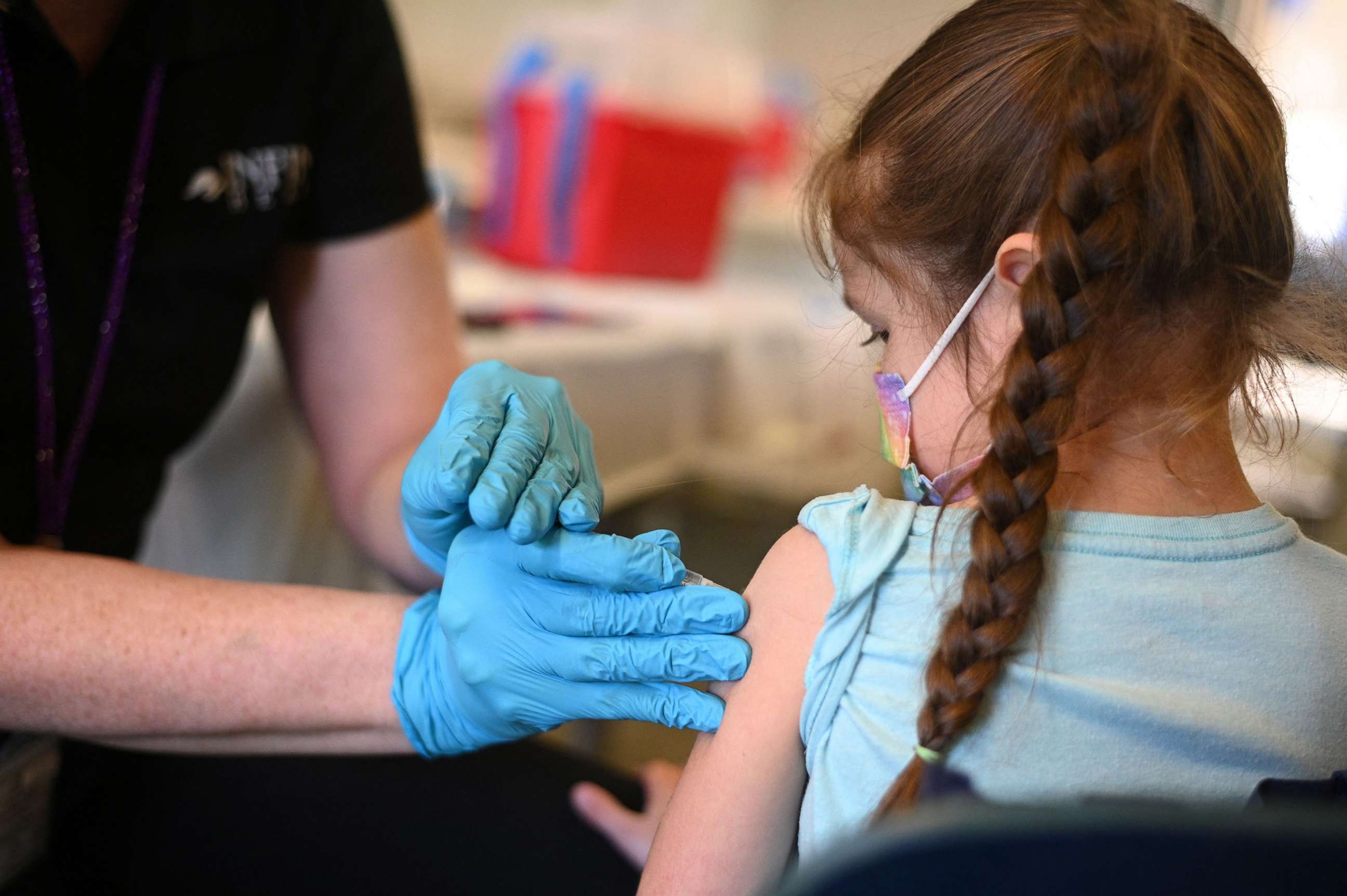
Moderna, which is a two-shot vaccine, is different from Pfizer’s vaccine, which is a three-shot vaccine. Pfizer hasn’t finished gathering its data yet, but some expect it to be more effective because booster shots, or third shots, have shown to boost immune response in adults.
Pfizer’s CEO Albert Bourla has said that he expected the company to submit data to the FDA in the “coming weeks,” and that it could be authorized in June.
And while Moderna has requested an emergency use authorization for its vaccine, potentially putting it ahead of Pfizer for timing, the company still must continue to submit more data for its full application over the next week or so.
The FDA has also signaled that the Moderna submission will take some time to sift through because it has to also review the company’s data on its vaccines for kids up to age 17, which haven’t been authorized yet.
Moderna, for its part, has said that it hopes its vaccine will be authorized within a month, which is the usual timeline for vaccine authorization during the pandemic, and that the FDA might prioritize reviewing the youngest age group before looking at the data from the older age groups, since there is no vaccine for kids under five.
"I think for these little children, they really represent an unmet medical need," Paul Burton, chief medical officer for Moderna, told ABC News on Wednesday. “I would be hopeful that the review will go on quickly and rigorously — but if it's approvable, this will be made available to these little children as quickly as possible."
Burton said that the FDA should have the bulk of the data it needs to do the review.
Moderna’s trial found that the shots generated a strong immune response with no significant risks. The vaccine generated an antibody response roughly equivalent to the antibody response seen in adults, the company said.
At the same time, experts have questioned the low efficacy numbers against infection. During the omicron surge, two doses of the vaccine were roughly 51% effective against COVID-19 infection, including asymptomatic and mild infections, for children 6 months to 2 years old, and 37% effective among kids 2 years to 6 years old.
But Burton, with Moderna, defended the vaccine’s efficacy against infection, arguing that omicron led to more breakthrough infections, but that the shot produced an antibody response that was even stronger in the young kids than it was in the 18-24-year-olds.
“I think moms and dads and caregivers, doctors and nurses should be reassured by this result,” Burton told ABC News.
“The antibody levels that we saw here were high, and we can translate that to what we see in adults where we get really good protection against severe disease and hospitalization,” he said.
Moderna is also studying third shots across all age groups, including for a variant-specific vaccine that could more effectively target some of the newer strains of the virus.