FDA authorizes updated COVID-19 boosters for kids under 5
The U.S. Food and Drug Administration authorized bivalent COVID-19 boosters for children between the ages of 6 months and 4 years old Thursday.
The booster, available from both Pfizer and Moderna, was previously authorized for everyone 5 years and older in October.
"More children now have the opportunity to update their protection against COVID-19 with a bivalent COVID-19 vaccine, and we encourage parents and caregivers of those eligible to consider doing so -- especially as we head into the holidays and winter months where more time will be spent indoors," FDA Commissioner Robert Califf said in a statement.
"As this virus has changed, and immunity from previous COVID-19 vaccination wanes, the more people who keep up to date on COVID-19 vaccinations, the more benefit there will be for individuals, families and public health by helping prevent severe illnesses, hospitalizations, and deaths."
The updated booster targets BA.4 and BA.5, which are subvariants of the omicron variant.
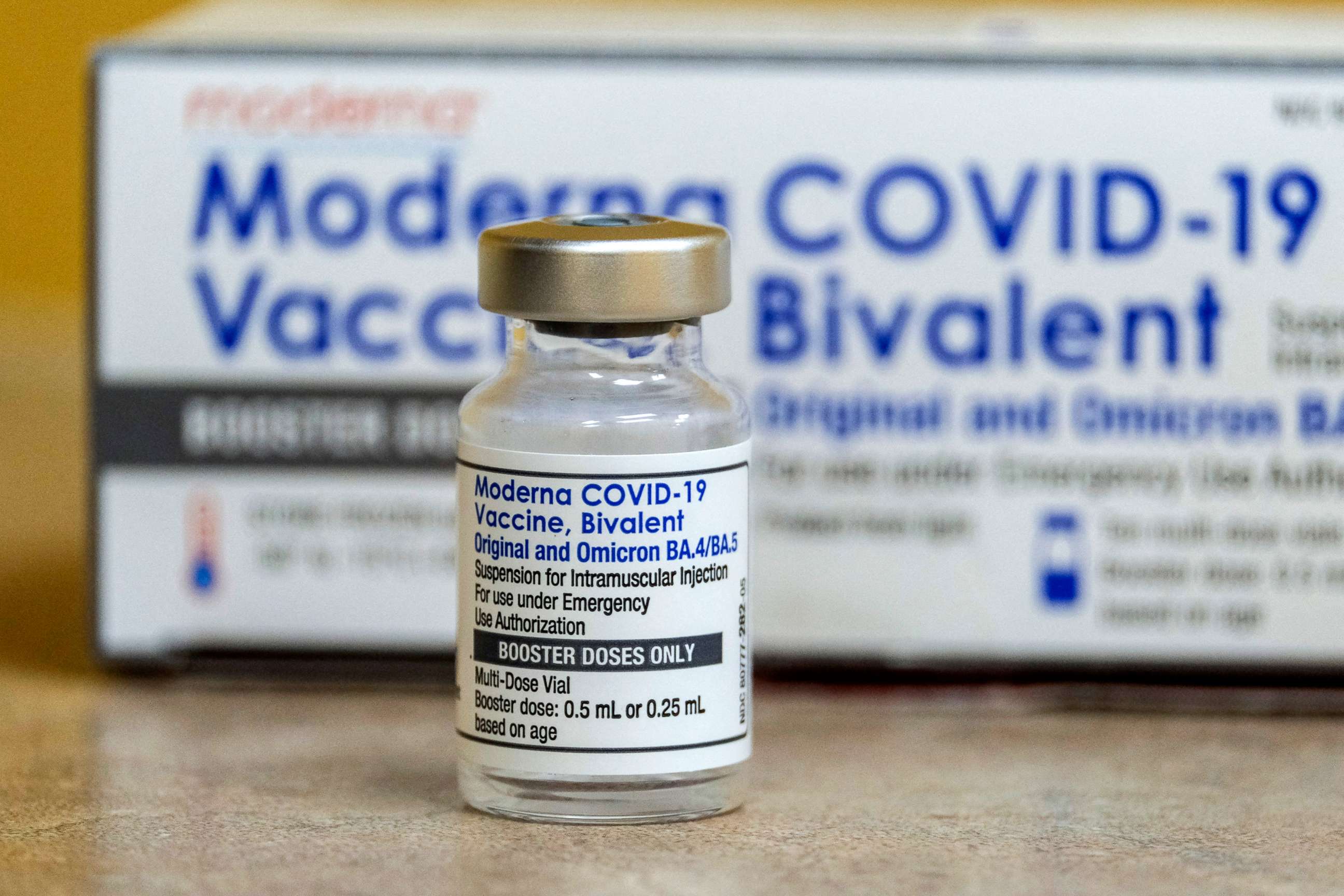
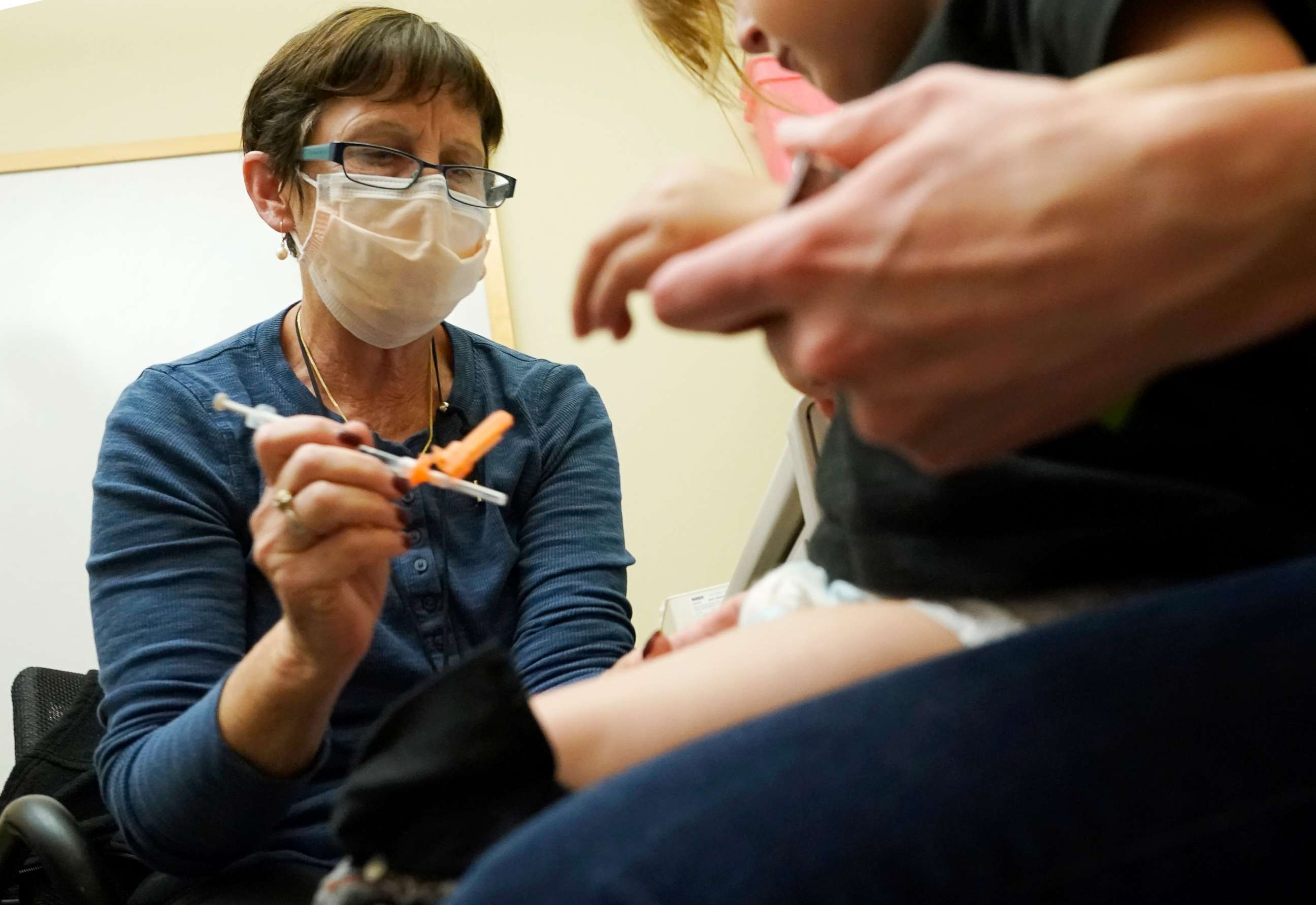
Children are eligible to receive the booster at least two months from the completion of their primary series or after receiving a separate booster dose.
The authorization means there are now more than 267 million people eligible for the booster in the U.S., according to data from the Centers for Disease Control and Prevention.
However, as of Nov. 30, only about 13% of those eligible have received an updated booster dose.
The final step before the booster can be distributed to the under age 5 category is authorization from the CDC, which must be signed off on by the agency's director, Dr. Rochelle Walensky.
When considering authorizing the updated booster, the FDA said it looked at immune response data from adults who had received the original booster, which targeted the original omicron variant BA.1, as well data from a study comparing children who received the original booster and children who only received the primary series.
"Vaccines remain the best defense against the most devastating consequences of disease caused by the currently circulating omicron variant, such as hospitalization and death," Dr. Peter Marks, director of the FDA's Center for Biologics Evaluation and Research, said in a statement. "Based on available data, the updated, bivalent vaccines are expected to provide increased protection against COVID-19."
"Parents and caregivers can be assured that the FDA has taken a great deal of care in our review, and we encourage parents of children of any age who are eligible for primary vaccination or a bivalent COVID-19 vaccine booster dose to consider seeking vaccination now as it can potentially help protect them from COVID-19 during a time when cases are increasing," the statement continued.




