CDC focused on finding 'nightmare bacteria' and preventing their spread: Report
Germs can cause infections. Germs can spread. Germs can battle back against antibiotics. And germs can share their weapons with other germs.
The Centers for Disease Control and Prevention communicated on Tuesday the early outcomes of their targeted campaign to identify infections by “nightmare bacteria” – any of several germs that cause infections that are nearly impossible to treat with currently available medications. These germs have defeated all current forms of antibiotics, which leaves doctors and hospitals almost helpless to fight their infections.
Labs in the 50 states and Puerto Rico tested 5,776 selected samples of highly resistant germs for unusual patterns of resistance. One out of every four bacterial samples was found to not only to have some antibiotic resistance, but to carry a gene capable of spreading that resistance. What surprised investigators most was that 221 of these had resistance genes described as “especially rare.” In the next step, CDC officials and local health care providers looked for “colonization” – they tested people in close proximity to the infected person (people who did not have infection themselves) to see if any were harboring the bacteria. Those who harbor an infection-causing organism but do not have an infection themselves are called “carriers.” One in 10 people tested were found to be carriers of the resistant bacteria, and many different species were detected.
This was the first nationwide testing of this magnitude searching out resistant bacteria and their genes. This was only the first part of a focused CDC initiative to address this progressively worrisome health scare.
What should you know about resistant bacteria? There are many classes of “microbes” or microscopic organisms in our environment: bacteria, viruses, fungi, and parasites. Within these groups, many of the organisms cause no problems (or can even be beneficial) to humans. Microbes that cause disease are sometimes referred to as “pathogens” or more simply as “germs.”
The development of antibiotics to treat pathogen bacteria was a true medical breakthrough. We have been treating infections with antibiotics for over 70 years, and common infections that used to kill are now trivial, since antibiotics fight the bacteria so well. Use of antibiotics decade after decade, though, can make them less effective in the long term.
One reason there are so many antibiotics is that some have always been more effective against certain types of bacteria. You can think of these the same way you think of the many different specialized weapons available to the military for different types of combat.
The concern, however, is that some antibiotics that used to work against certain species of bacteria no longer work in certain strains of that species. And the list is growing. This occurs because bacteria can learn to put up a defense against the antibiotic. This shows up when a bacteria is exposed to an antibiotic it has seen before – or even one similar to it. The bacteria, in essence, has altered slightly, and now can avoid attack by the antibiotic. These “resistance” systems can be incredibly diverse, complex, and even be described as creative. When bacteria “resist” the drug, the germ is not killed as intended, the infection is not treated, though the antibiotic may kill other, less important, bacteria, giving the resistant bacteria free rein to multiply and spread further.
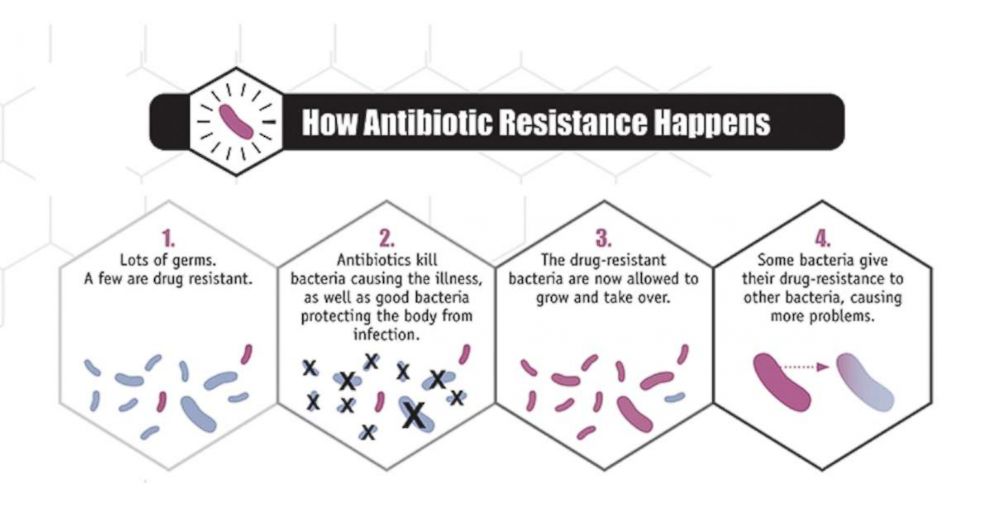
Medical laboratories can test bacteria samples from a patient to determine which antibiotics the bacteria likely will – or will not – respond to. This is called a “resistance pattern.” When a lab finds that a germ is resistant to a class of antibiotics that are typically a good match for that organism, this is called an “unusual resistance pattern” and it can signal bad news – the potential for this germ to become resistant to all, or nearly all, antibiotics in existence.
Some germs gain nicknames based on the major antibiotic they are resistant to: MRSA stands for “methicillin-resistant staph aureus” (this strain has acquired resistance to methicillin, which is a stand-in for all drugs in the penicillin family). CRE stands for “Carbapenem-resistant enterococci”, indicating resistance to one of the more powerful classes of antibiotics available.
A bacteria resistant to multiple classes of powerful antibiotics is “multi-drug resistant.” As Dr. Anne Schuchat, Principal Deputy Director of CDC, describes, these germs cause “resistant infections that are virtually untreatable with modern medicine.” Certain individuals are more likely to get these infections: people who live in nursing homes or other long term care facilities, or those are frequently hospitalized, are at high risk because they live in a setting where many people with infections are close together and germs can be easily shared. They also tend to have a weaker immune system to fight infections when they occur. Young adults with cystic fibrosis are another at-risk group. They have many respiratory infections in their lifetime requiring many courses of antibiotics – and many become carriers of highly-resistant bacteria as a result.
Sometimes the resistant bacteria can be treated with more powerful or less commonly used antibiotics – which can have more toxic effects and be extremely costly to the health care system. Sometimes the only options for treatment are what doctors call “supportive care,” providing measures like oxygen, medications to support blood pressure, or even ventilator or dialysis machines to support failed organs, hoping that the body’s immune system will find a way to fight the infection. Despite these measures, up to 50 percent of nightmare bacteria infections result in death.
If that’s not bad enough, scientists also have learned that germs are doing the equivalent of crowd-sourcing – one bacteria that develops resistance to an antibiotic can “share” the information for that defense system, packaged in DNA that can be passed from one cell to another. Resistance genes, or pieces of DNA, turn regular germs into nightmare bacteria. These genes are the focus of the current CDC effort.
What can be done to stop the spread? Exposure to antibiotics (“studying the enemy”) is how bacteria develop their resistance tools. For that reason, physicians know that antibiotics should only be used when they are needed and antibiotics (which specifically treat germs in the bacteria classification) should NOT be used in an attempt to treat infections caused by other germs -- like viruses. For instance, almost all “common cold” infections are viruses, yet patients want to be given something, and often insist on an antibiotic, though it has no chance of affecting the virus. As Dr. Jay Butler described, providers aim to use antibiotics in the “right clinical situations with the right drug at the right dose for the right duration.”
According to the CDC, up to 50 percent of antibiotic prescriptions violates one of these principles.
Another way that antibiotic resistance has spread is through widespread use of antibiotics in food-producing animals for the purpose of accelerating animal growth. Per the CDC website, “antibiotics…should be used in food-producing animals only under veterinary oversight and only to manage and treat infectious disease, not to promote growth.”
What is the CDC doing? In 2016, the CDC launched a landmark antibiotic resistance solutions initiative, dedicating more resources than ever before to preventing the spread of resistant bacteria. The two major features of this initiative are rapid identification of unusual resistance patterns among infection-causing bacteria, and standardized infection control measures in the involved health care facilities, including finding individuals colonized with the same germ and intensifying the steps taken to prevent spread of the most dangerous organisms.
Prevention of infections and the spread of bacteria isn’t a new concept. What is new, however, is the resources available to physicians and other health professionals to do it. Labs have more capacity to test for the presence of germs that carry resistance and get these results quickly. Dr. Schuchat said that the CDC is now “encouraging health care facilities and public health authorities to respond to even single cases of an emerging antibiotic resistant pathogen.” The analogy used by the CDC is that of a fire. In the past, public health authorities have had to dedicate their resources to fighting the wildfires - the most resistant and most widespread of the nightmare bacteria that are already out of control. This naturally has allowed for the development of even more resistant bacteria. With the increased funding available, authorities can respond to many more small "fires" and even "sparks," to prevent further spread.
In addition to additional funding, the CDC is sending 500 more local staff to partner with facilities around the country when unusual resistance patterns are detected. They described one recent example, when an Iowa nursing home resident had a urinary tract infection. The CDC Antibiotic Resistance Lab Network performed rapid testing which showed unusual resistance. The Iowa department of health worked with the nursing home to do site assessments and identify ways the germ might have spread. They tested other residents in this facility for colonization and found that 5 others were carrying the dangerous bug. They implemented aggressive infection control measures and continued testing patients until the bug was no longer detected.
Obviously this is very time- and resource-intensive, which explains why this approach is best applied to germs that are potentially very dangerous. As the CDC leadership pointed out, however, this is more effective (and less costly) if the tools are there to step in immediately after a single case is detected, rather than wait for an outbreak. Per Dr. Schuchat, “These unusual threats are the uncommon or highly resistant germs that have yet to spread throughout the U.S. We are working to get in front of them before they do become common in order to protect patients now and in the future.”
Dr. Jay Butler, Chief Medical Officer and Director of Alaska Division of Public Health and the Association of State and Territorial Health Officials (also known as ASTHO), put it another way: “We can’t wait until one case becomes ten or ten cases becomes a hundred. We can intervene early and aggressively to stop spread and to keep these threats out of our states.” He added, “I have heard from my state and local colleagues from across the nation that these resources have been a game changer in their states.”
The work is just beginning. For each resistant bacteria stifled, another is intensifying its own resistance. “We need to do more and we need to do it faster and earlier with each new antibiotic resistance threat,” Dr. Schuchat said. Health care providers and public health officials alike are now more equipped than ever to do just that.
Dr. Kelly Arps is a resident physician in internal medicine at Johns Hopkins Hospital. Kelly is working with the ABC News Medical Unit.