Blood pressure medication recall: What you need to know
The Food and Drug Administration has recently recalled a number of blood pressure medications after discovering that they contained potential cancer-causing contaminants.
In the latest case, detailed on Monday, Torrent Pharmaceuticals Limited expanded its voluntary recall for losartan potassium tablets and the combination medication, losartan potassium/ hydrochlorothiazide tablets. Other lots of losartan as well as other drugs from several manufacturers have been recalled voluntarily in recent months as well due to contamination from other chemicals.
The drugs from Torrent, with lot numberslisted on the FDA website were believed to be contaminated with "unacceptable" amounts of N-methylnitrosobutyric acid or NMBA, a potential human carcinogen.
Losartan, a blood pressure medication in the angiotensin II receptor blocker (ARB) class, helps reduce blood pressure by preventing blood vessels from narrowing or constricting.
A rep at Torrent Pharmaceuticals Limited said that "no adverse events have been reported yet."
Per the FDA website, people taking these medications are encouraged to continue taking their medication "as the risk of harm to the patient’s health may be higher if the treatment is stopped immediately without any alternative treatment." They are asked to consult their physicians or pharmacists before making any changes to their medication regimen.
Torrent Pharmaceuticals Limited said it suspects that contamination is related to manufacturing at a specific site and is therefore working on "manufacturing and distributing new pills without the contaminant."
The rep urged that anyone who is taking these medications should call Torrent Pharmaceuticals Limited at 1-888-280-2040. The company has shared that they will take back contaminated medications and provide refunds.
FDA has recalled multiple blood pressure medications
Over the past year, the FDA has recalled multiple blood pressure medications from several manufacturers after discovering potential carcinogens in some batches. It’s believed they became contaminated during the manufacturing process.
The recalled medications include specific “lots” of losartan, irbesartan, valsartan and combination drugs with valsartan. The investigation is ongoing and the FDA continues to update the list of medications being recalled here.
Some of the recalled lots contain a combination of medications. Although neither amlodipine nor hydrochlorothiazide are currently under recall on their own, they are when combined with some other medications. For example, a combination of valsartan, amlodipine and hydrochlorothiazide is one product that has been recalled. The FDA website provides lists of valsartan products under recall, valsartan products not under recall, irbesartan products under recall and losartan medications under recall for patients, health care providers and pharmacists to accurately confirm which products are affected.
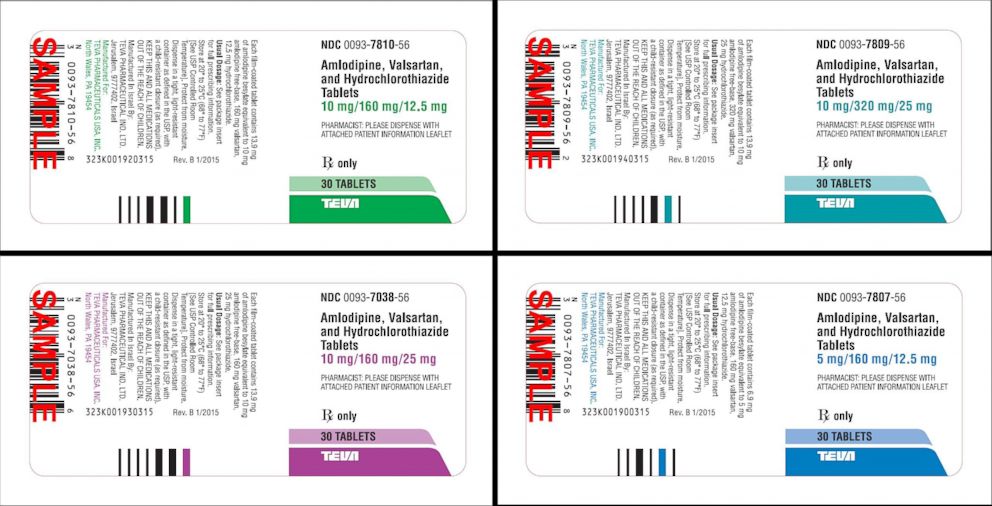
Those voluntary recalls came after the discovery of the chemicals NDMA and NDEA -- both probable human carcinogens -- at levels far above what the FDA considers an acceptable daily intake. NDEA is a naturally occurring chemical in some foods and it can be found in drinking water, but it’s also present in air pollution and industrial processes. NDMA is a chemical that forms in both industrial and natural processes.
With that said, here are some things you should know if you’re taking these medications.:
You shouldn’t stop taking the medication until you speak to your doctor first.
To date, none of the manufacturers have received any reports of people who use these medications becoming ill. If you’re taking a blood pressure medication that was recently recalled, do not stop taking it.
The FDA says that “the risk of harm to someone’s health may be higher if one of these medications is stopped immediately without any alternative treatment.”
So, if you’re taking one of these medications, reach out to your pharmacist or physician to discuss alternative treatments.
These medications will lower your risk for heart disease.
These medications are for high blood pressure and fall in a drug class known as angiotensin receptor blockers (ARBs). They work by blocking the production of a natural hormone called angiotensin II, which narrow your arteries, raising blood pressure. High blood pressure, which is also called hypertension, causes stress on your heart and can contribute to heart disease.
High blood pressure affects 75 million American adults.
High blood pressure is one of the most common medical conditions. Blood pressure is a measure of how efficiently our hearts push blood through our arteries. If the pressure is too low, there is a risk that organs will not get enough blood. If the pressure is too high, there’s a risk that the heart will work too hard.
Normal blood pressure fluctuates.
Blood pressure measurements are actually two measurements: systolic, which is when the heart squeezes blood through the body, and diastolic, which is the pressure of blood when the heart is at rest.
If your doctor says your blood pressure is 130/80, the 130 would be systolic and the 80 would be diastolic.
While your target blood pressure is often determined by your age and medical conditions, most doctors recommend keeping it around 120/80.
Several reasons people get high blood pressure and why it’s normal for it to fluctuate
It’s important to understand that your blood pressure measurement will vary throughout the day; it’s normal. High blood pressure can develop for several reasons, including having a family history, obesity, smoking, high-salt diets, diabetes, lack of exercise and underlying kidney disease. Often, it can be controlled with a combination of lifestyle modifications and a variety of medications.
Tulsie N. Patel, MD is a chief resident in psychiatry from Dallas, TX, working with the ABC News Medical Unit. Dr. Sumir Shah is an emergency medicine physician and Dr. Saumya Dave is a resident physician in psychiatry and they both are members of the ABC News Medical Unit which can be reached @ABCNEWSHealth.