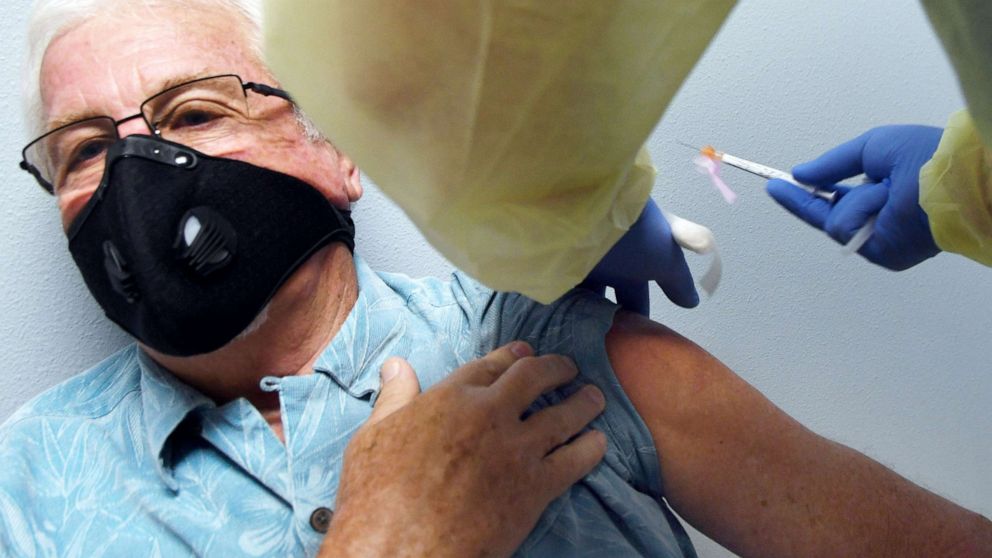
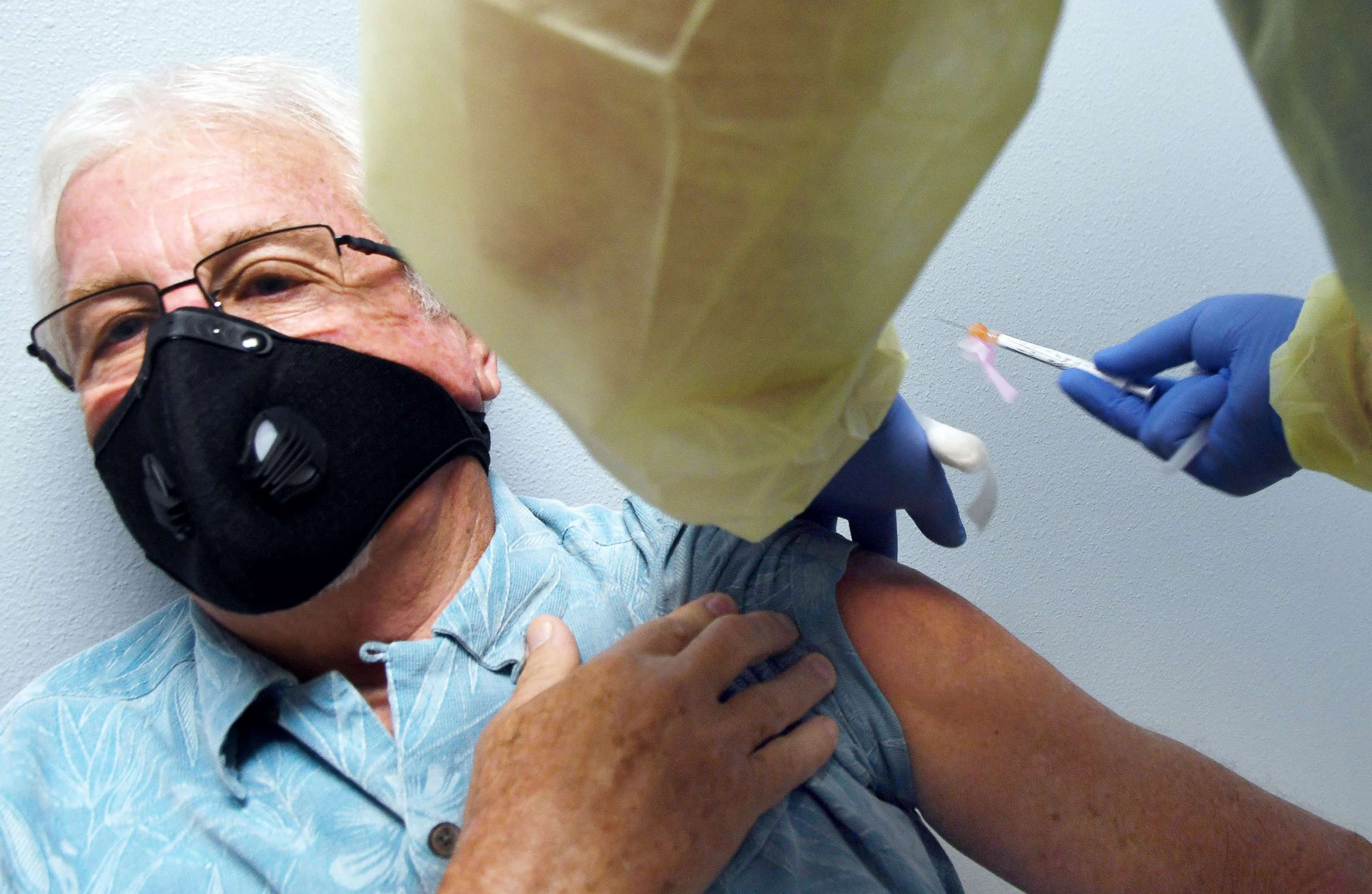
Panel recommends Moderna vaccine, paving way for FDA authorization
Federal advisers voted overwhelmingly Thursday to recommend the Moderna vaccine for people over the age of 18, clearing a path for government authorization on what would become the nation's second vaccine to prevent COVID-19.
The panel of independent advisers, called the Vaccines and Related Biological Products Advisory Committee, found that based on evidence of a clinical trial involving 30,400 people, the Moderna two-dose vaccine is safe and likely more than 94% effective in preventing serious illness caused by the virus.
The decision paves the way for a final green light from the U.S. Food and Drug Administration, which in turn triggers the shipping of 5.9 million doses.
"All these vaccines will not be the magic bullet and miraculously reverses all the damage this pandemic has caused. It gives us hope that one day in the not-too-distant future, some semblance of normalcy will be within our reach," said Robert Wong, a physician at the Veterans Affairs Palo Alto Healthcare System, who spoke during a public comment session.
Wong urged the panel and federal regulators to be "particularly cognizant" of ensuring equitable access among underserved and vulnerable populations that "have been disproportionately exacerbated by this pandemic."
The independent panel of advisers was asked to vote on this question: "Based on the totality of scientific evidence available, do the benefits of the Moderna COVID-19 vaccine outweigh its risks for use in individuals 18 and older?"
The panel voted in favor, 20-0, with one abstention, to recommend the vaccine.
The questions of allergies were raised, following reports of rare reactions to the Pfizer vaccine, including two in Alaska on Wednesday.
Dr. Doran Fink, a senior vaccine official at the FDA, said researchers didn't have all the information. But he noted that "these cases underscore the need to be vigilant during the early stage of the campaign" and communicate those findings to the public.
Fink said the FDA was working with Pfizer to further revise fact sheets and warnings to health care providers to make clear that any facility administering it "should ensure that medical treatment for managing serious allergic reactions is immediately available." He said the FDA plans to do the same for Moderna, if it is ultimately authorized as expected.
If authorized, the 5.9 million doses shipped from Moderna would be in addition to the 6.4 million doses provided by Pfizer-BioNTech that started to roll out this week after being the first to get emergency use authorization.
Dr. Anthony Fauci, the nation's top infectious disease expert, said Wednesday that people shouldn't be concerned about having to choose between Moderna and Pfizer. An internal assessment by the FDA already found that the Moderna data show the benefits likely outweigh the risks.
"Moderna certainly will be available soon and have the same level of efficacy ... the same safety profile and virtually everything the same about that. So it wouldn't make much difference which of those you take," Fauci told CNBC's "Health Returns."
One primary difference is that Moderna requires fewer specific handling instructions because it does not require ultra-cold storage conditions like the Pfizer-BioNTech vaccine.
Moderna also is seeking authorization for ages 18 and older, whereas Pfizer's enrollment of older teens in trials this fall paved the way for authorization of anyone 16-plus.
Which sites get the first doses are being decided by state governors and health departments. As recommended by an ethics board, hospitals have been first in line, injecting front-line workers most likely to come in contact with the virus.
Nursing homes also are the priority, although they have been slower to roll out shots because they rely on CVS, Walgreens and other pharmacies that say the logistics are considerable to ensure no dose is wasted.
Florida and West Virginia were among the first to host vaccine clinics at nursing homes on Wednesday, with a nationwide rollout at long-term care facilities expected on Monday.
By February, it's expected that other seniors living independently – anyone over age 65 – will be able to ask their pharmacy for a vaccination.
"Our plans is to be able to immunize 100 million of these people (with) those vaccines by the end of the first quarter of the year 2021, somewhere during the month of March," Moncef Slaoui, Trump's top science adviser in the vaccine effort, told CNBC.
A third vaccine also is on the horizon. Johnson & Johnson was expected by early January to know whether its vaccine was effective. If that vaccine comes online as well, that "will help us accelerate even faster coverage of that population," Slaoui said.
Estimates for the broader population remains March or April, with immunizations complete by June.
"By the time we get into mid, fall of 2021, we can be approaching some level of normality," he said.
"I would think that would be things like being able to go to theaters, clearly feeling much more comfortable about school, having restaurants open to indoor dining," Fauci said.
This report was featured in the Thursday, Dec. 17, 2020, episode of "Start Here," ABC News' daily news podcast.
"Start Here" offers a straightforward look at the day's top stories in 20 minutes. Listen for free every weekday on Apple Podcasts, Google Podcasts, Spotify, the ABC News app or wherever you get your podcasts.