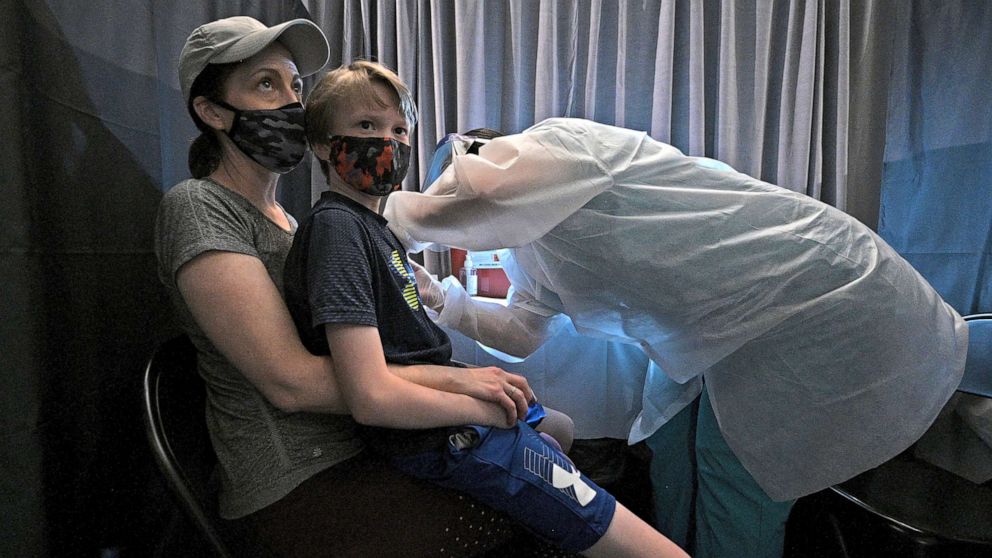
Pfizer advances clinical trials for 5- to 11-year-olds at lower doses
Pfizer is advancing Phase 2/3 clinical trials for young kids at lower doses than vaccines for adults, the pharmaceutical company announced Tuesday.
Based on safety, efficacy and tolerability data from Pfizer's Phase 1 trial, the company will use 10 micrograms of each vaccine dose for kids between the ages of 5 and 11 in Phase 2/3 trials, and 3 micrograms of each dose for those 5 and younger. People ages 12 and older received 30 micrograms in each dose.
In addition to studying vaccine safety and side effects in kids, the study will examine antibody response to ensure the vaccine produces enough immunity at lower doses.
The study will include up to 4,500 participants from the United States, Finland, Poland and Spain, according to Pfizer.
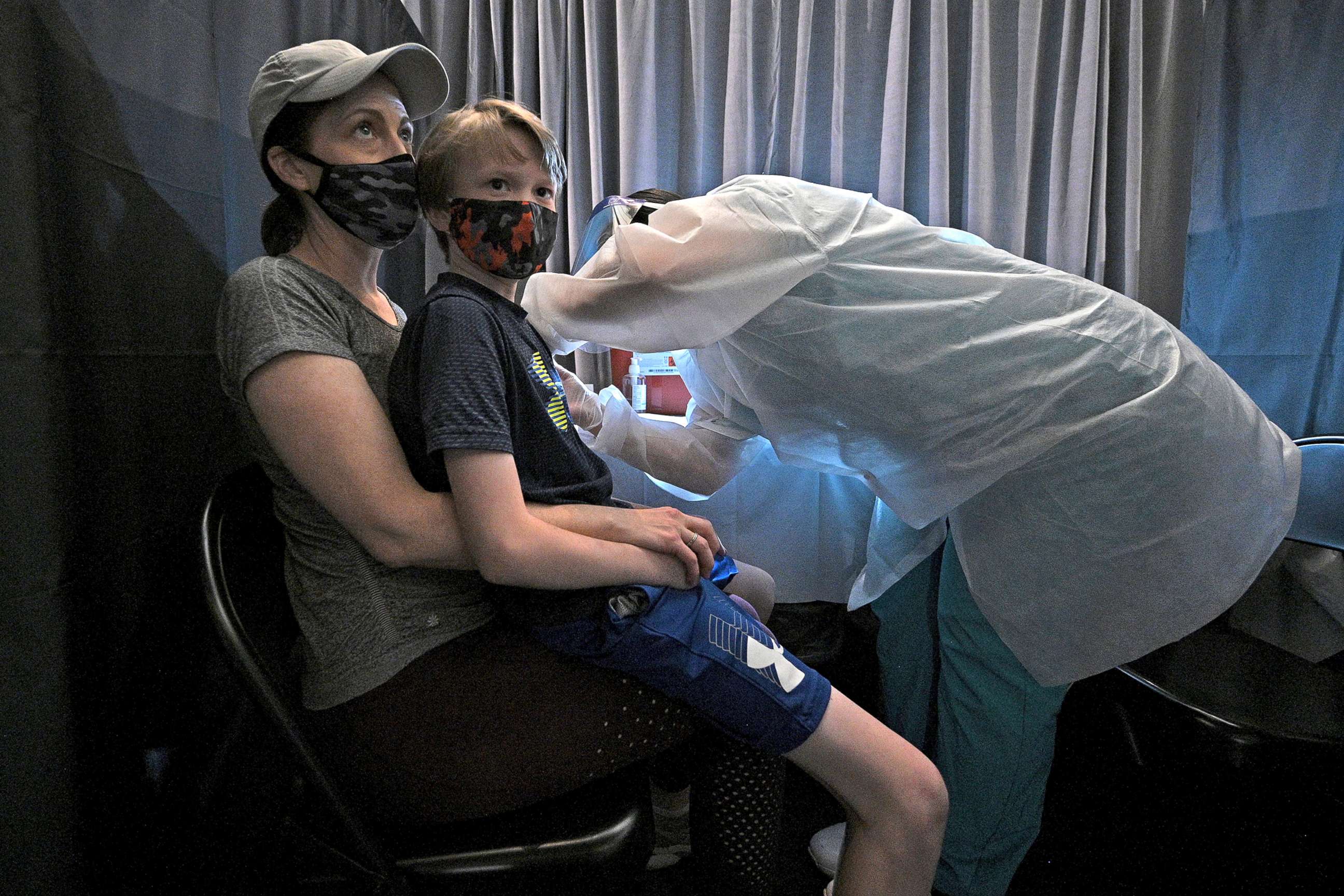
"Today marks an important next step in our efforts to understand the safety and immune response of our COVID-19 vaccine," Dr. Bill Gruber, senior vice president of clinical research and development at Pfizer, said in a statement. In the coming weeks, Phase 2/3 trials will start for 2- to 4-year-olds, as well as those as young as six months, according to Gruber.
"We take a deliberate and careful approach to help us understand the safety and how well the vaccine can be tolerated in younger children," Gruber said.
"Children younger than 12 make up a significant portion of the total global population and can develop COVID-19 disease, and also can spread the virus to others," Gruber added. "If successful, we believe vaccinating children will help further protect our communities and contribute to the evolving herd immunity.”
Pfizer anticipates having initial results of the Phase 2/3 trials in September for the 5- to 11-year-old group, with results for kids of 2 and 5 expected shortly after that. Results for children between the ages of six months and 2 years old are expected in October or November, according to a Pfizer spokesperson.
In every instance, Pfizer would potentially apply for emergency use authorization in the U.S. shortly after getting results, assuming they showed the vaccines to be safe and effective in those age groups at those doses.
ABC News' Eric Strauss and Sony Salzman contributed to this report.