Contaminated eye drops linked to more deaths as 14 people report vision loss
An additional person has died in an outbreak linked to contaminated eye drops and more people are reporting they've lost their vision.
The number of deaths has risen to four, according to an update issued by the Centers for Disease Control and Prevention on Friday and first reported by ABC News.
At least one of the deaths occurred in Washington state, but the CDC did not provide any information on the other victims.
Additionally, at least 14 people have gone blind, up from eight reported during the last update in March. Four people have had their eyeballs surgically removed but that number has not risen.
Patients reported using at least 10 different brands of artificial tears but most cases have been linked to EzriCare and Delsam Pharma eye drops, made by India-based Global Pharma Healthcare.
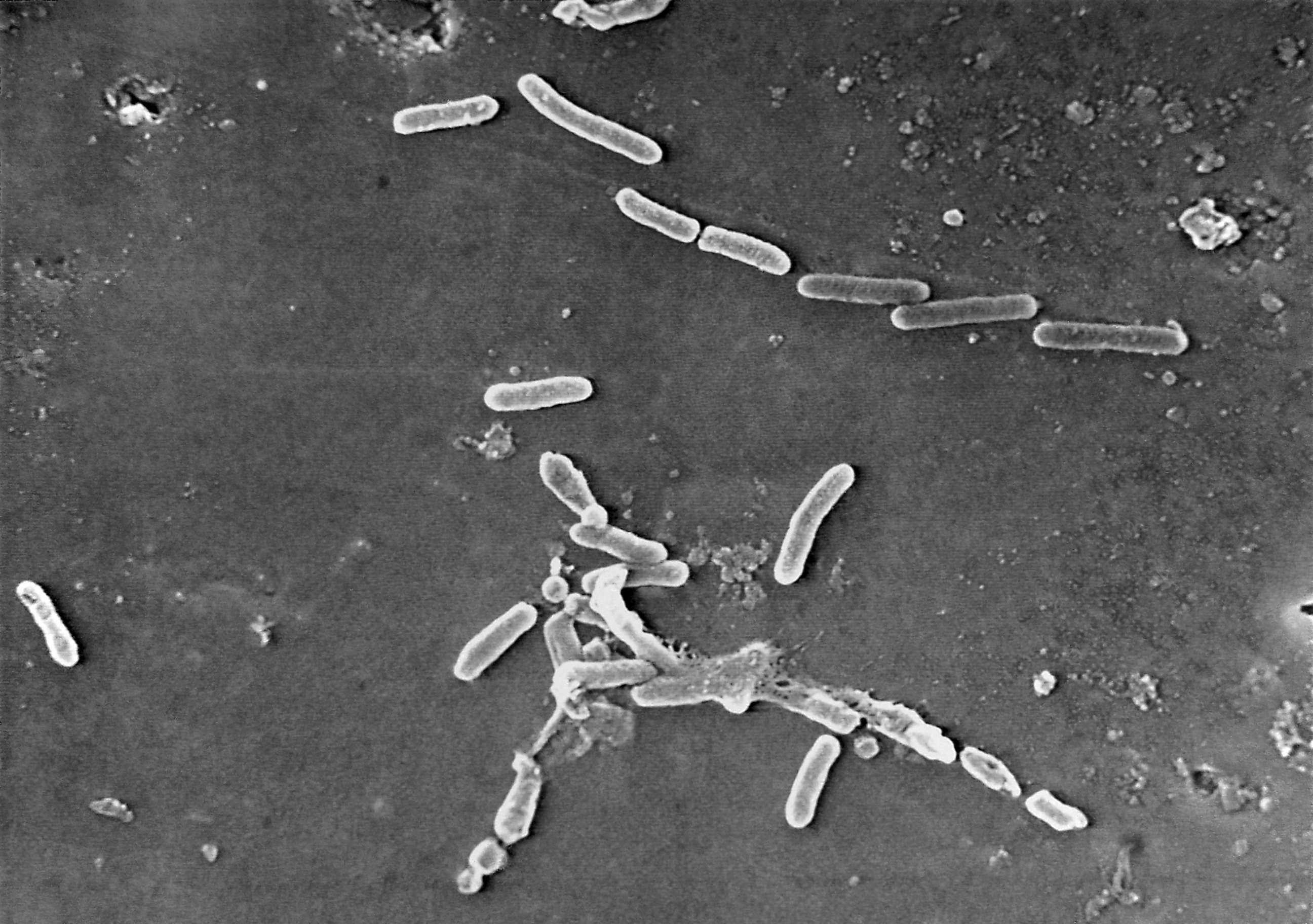
The eye drops were contaminated with an antibiotic-resistant form of Pseudomonas aeruginosa, an aggressive bacterium, according to the CDC.

Pseudomonas are a type of bacteria found in the environment, with P. aeruginosa being the most common to cause infections in humans.
The infection is common health care settings and spreads from improper hygiene either due to unclean hands or medical equipment and surfaces not being properly cleaned.
P. aeruginosa is resistant to multiple types of antibiotics and has caused about 32,600 infections among U.S. hospitalized patients and an estimated 2,700 deaths, according to the CDC.
The strain that has been linked to the outbreak, however, had never been reported in the United States before, the CDC stated in its update.
As of May 15, 81 people across 18 states have been infected with P. aeruginosa, an increase of 13 patients since the last update.
Symptoms of their infections include yellow, green, or clear discharge from the eye; eye pain or discomfort; red eyes or eyelids; feeling of something in the eye; increased sensitivity to light; and blurry vision.
In February, the U.S. Food and Drug Administration issued a warning, backed by the CDC, urging clinicians and the public not to buy EzriCare Artificial Tears or Delsam Pharma's Artificial Tears due to potential bacterial contamination.
After the warning, Global Pharma Healthcare issued a voluntary recall of both products, notifying distributors and advising wholesalers, retailers and customers who have the products to stop usage. Global Health Pharma has also issued a recall of Delsam Pharma's Artificial Ointment.
The CDC and the FDA are warning that anybody who still has these brands immediately stop use and discard. None of the products appear to be able to be bought online.
Among the 13 new cases that were reported to the CDC, six had specimens collected prior to the February recall.
"These cases were confirmed after the recall date due to the time it takes for testing to confirm the outbreak strain and because of retrospective reporting of infections," the CDC wrote in its update.
Of the seven patients with specimens collected after the recall, they either were living in long-term care facilities with other known cases or were using a recalled brand of artificial tears.




