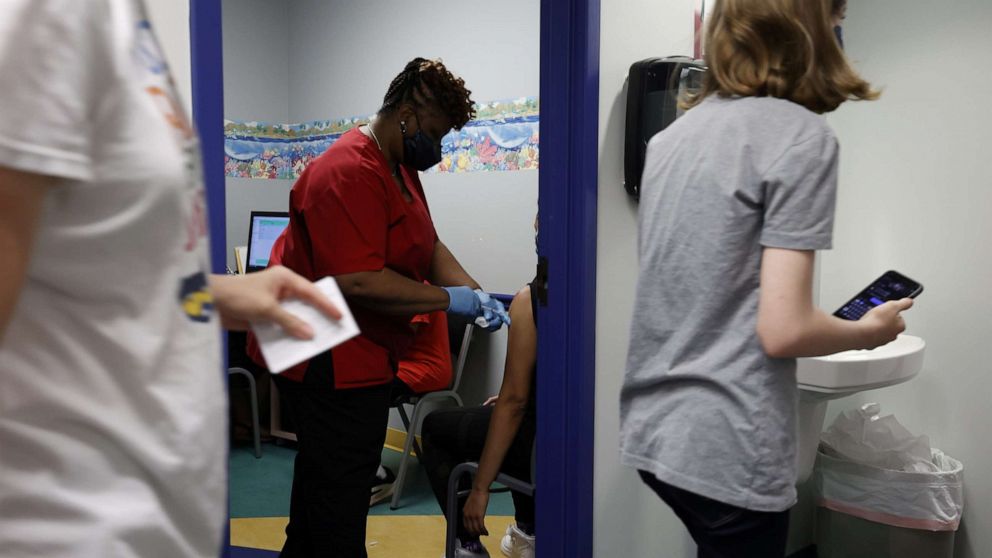
CDC panel votes to recommend vaccine for children ages 12 to 15
An independent panel of advisers to the Centers for Disease Control and Prevention voted Wednesday to recommend the Pfizer vaccine for use in children ages 12 to 15.
It marks a crucial milestone in the nation's push to tamp down COVID-19, with more shots in more Americans' arms helping pave the gradual path towards recovery and a return to normalcy. The Food and Drug Administration authorized the Pfizer vaccine for this age group Monday.
President Joe Biden hailed the decision in remarks on vaccinations Tuesday afternoon.
"My hope is that the parents will take advantage of the vaccine, and get their kids vaccinated," Biden said. "Let's remember that millions of 16- and 17-year-olds have been safely vaccinated and as more and more Americans are vaccinated, COVID-19 hospitalizations and death rates continue to fall."

Getting adolescents vaccinated was part of the plan he laid out last week with a goal of getting 70% of Americans vaccinated by July 4.
Biden touted the safety of the vaccines and also the increasing convenience of access to doses for those who want them.
"This new population is going to find the vaccine rollout fast and efficient," Biden said Tuesday. "As of tomorrow, more than 15,000 pharmacies across this country will be ready to vaccinate this age group."
CVS and Walgreens announced they would begin offering vaccinations for 12- to 15-year olds on Thursday.
Parental or legal guardian consent is required to receive the vaccine and children must be accompanied by an adult. Both companies encouraged people to make appointments.

CDC Director Rochelle Walensky signed off on the recommendation later Wednesday, meaning that vaccinating children as young as 12 is now the official recommendation by the nation's top public health experts.
While health experts said it's rare for children to get very sick with COVID-19, there is still a risk. Also, officials hope adolescent vaccinations will help lower the risk of transmission, as classrooms and summer camps both look towards reopening.
It comes as children now make up 22% of recent COVID-19 cases in the nation, according to the American Academy of Pediatrics and the Children's Hospital Association report.
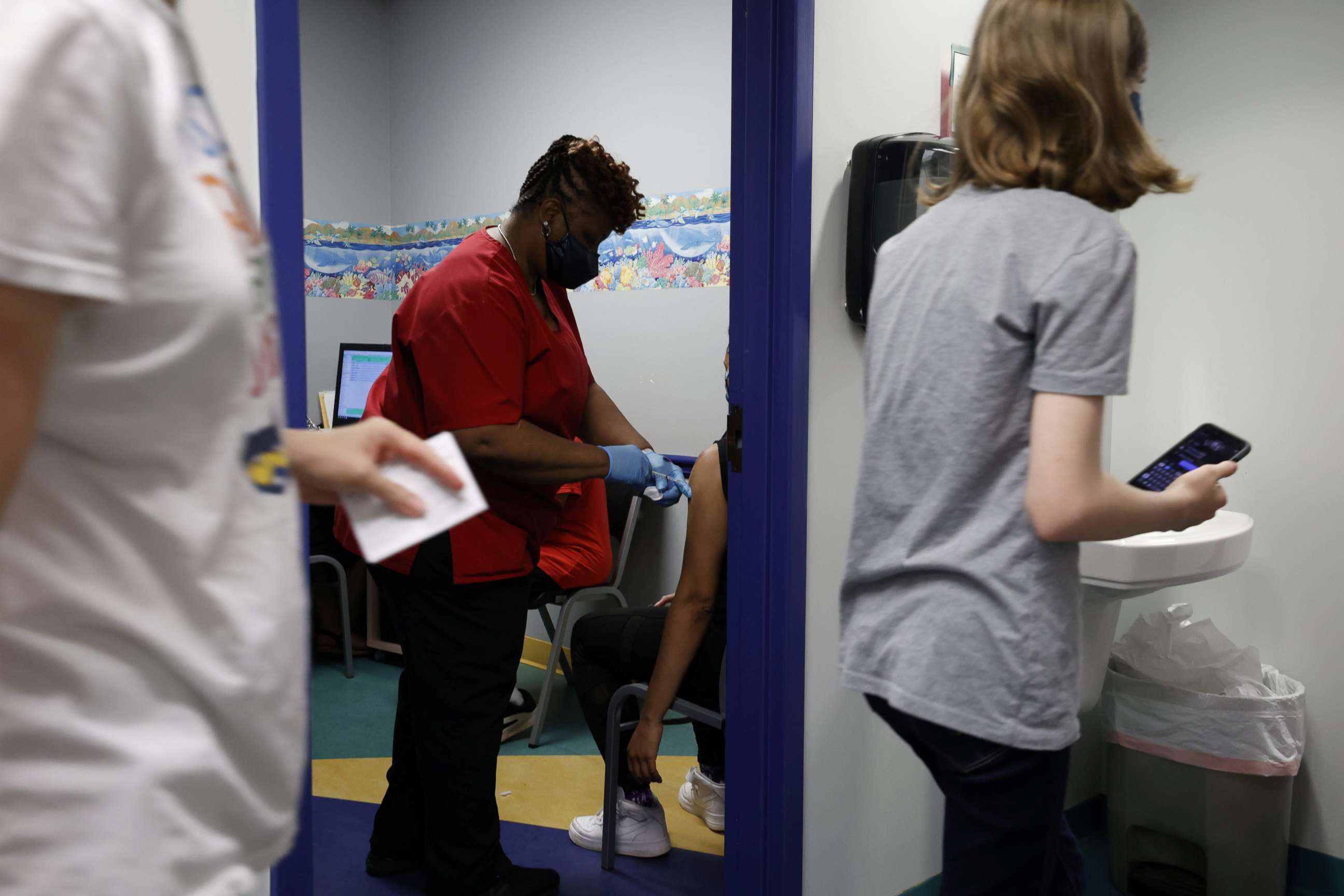
Pfizer's clinical trials have shown their vaccine safe and 100% effective in children ages 12-15, aligning with the 95% efficacy among adult clinical trial participants. Among the more than 1,000 adolescents who received the vaccine, there were no cases of COVID-19 recorded; there were 16 cases among the 978 participants who got the placebo.
On safety, clinical evidence showed no current safety concerns for these children receiving this two-dose vaccine, with side effects similar to those observed in adults -- like a sore arm at the injection site, fatigue and headache, which should stop within a few days of getting the shot.
Children who are fully vaccinated would be able to follow the updated guidance for vaccinated people from the CDC. That includes not having to wear masks outdoors unless in a crowded venue and not having to quarantine if there is a known exposure.